Andrea Markovinovic, PhD
Research
How do ER-mitochondria interactions and signalling regulate neuroinflammation in neurodegeneration?
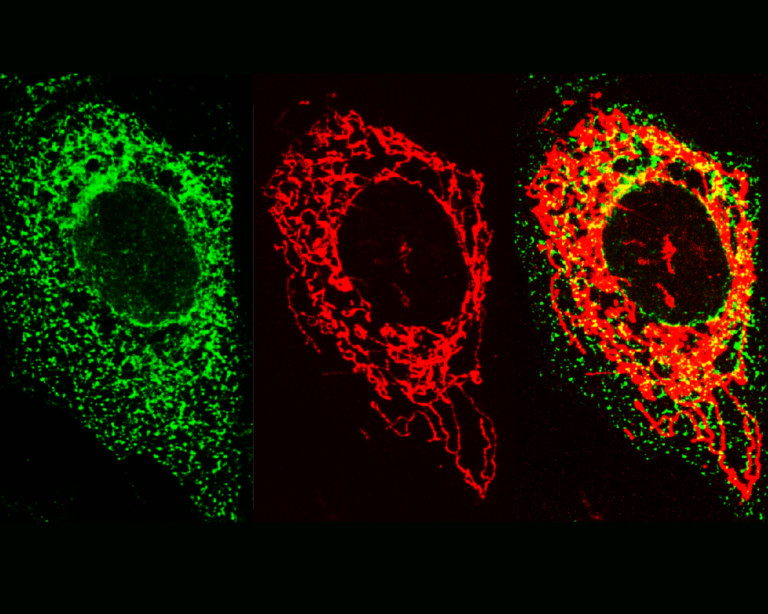
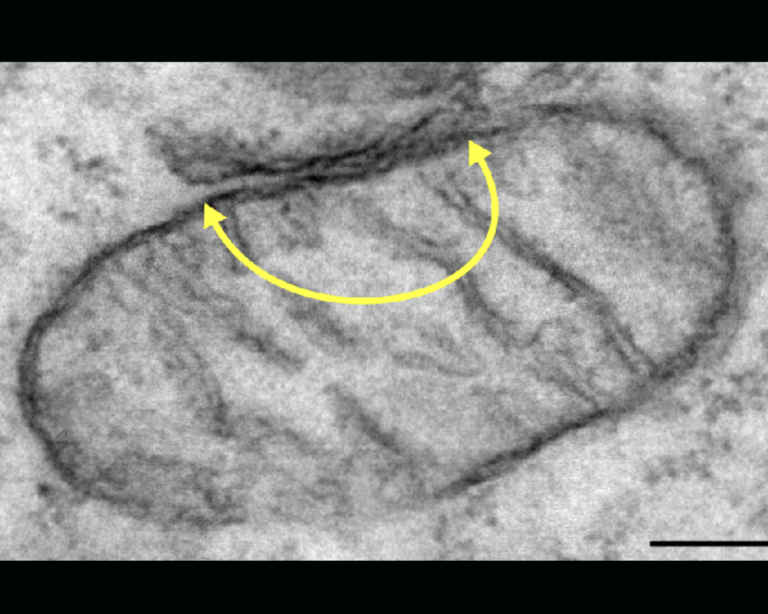
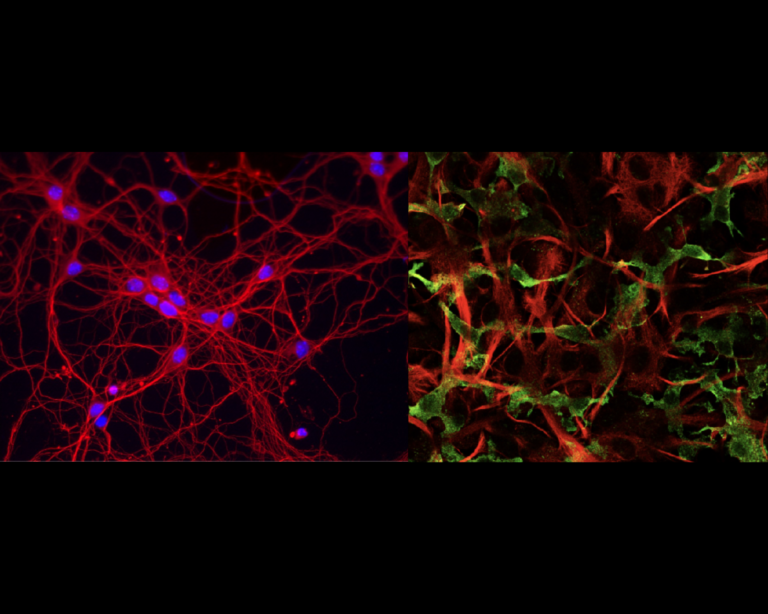
It is now well established that ER and mitochondria interact with one another to regulate number of physiological functions including mitochondrial function, ER stress responses, Ca2+ signaling, lipid synthesis, autophagy, synaptic function and inflammation. To facilitate this signalling, ER and mitochondria form close contacts through specialized ER regions called mitochondria-associated membranes (MAM). The mechanism by which MAM is recruited to the mitochondrial surface involves proteins that act to “tether” the two organelles. I am particularly interested in interaction between an integral ER protein VAPB and an outer mitochondrial membrane protein PTPIP51. There is increasing evidence that damage to ER-mitochondria signaling involving breaking of the VAPB-PTPIP51 tethering proteins contributes to neurodegeneration and this is especially the case for frontotemporal dementia/amyotrophic lateral sclerosis. The VAPB-PTPIP51 tethers are now known to regulate,
ER-mitochondria Ca2+ delivery and linked mitochondrial ATP production, lipid synthesis, autophagy and synaptic activity. However, we have no evidence on the role of VAPB-PTPIP51 interaction in neuroinflammation despite the evidence showing that ER-mitochondria contact sites are molecular platforms for the activation of key signalling pathways of innate immunity. Since neuroinflammation is one of the most consistent pathological hallmarks across neurodegenerative diseases, it is particularly important to address this lack of knowledge.